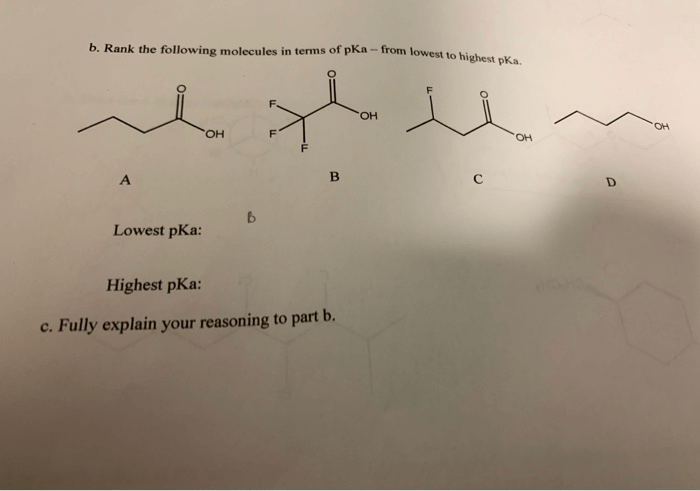
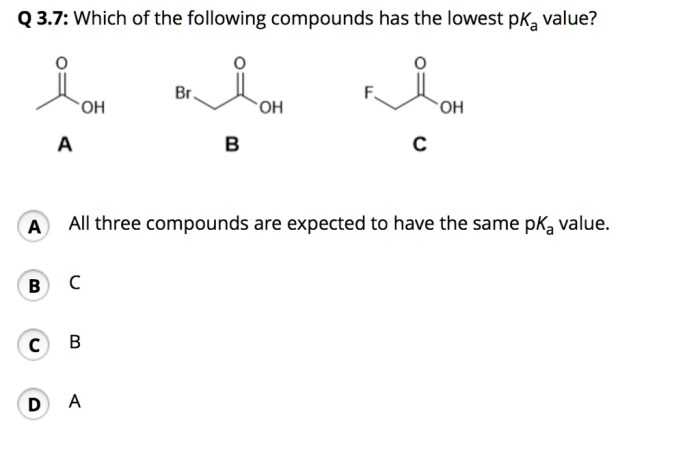
Which of the following compounds has the lowest pKa? This intriguing question sets the stage for an exploration into the fascinating world of acid-base chemistry. Understanding pKa, a measure of acidity, is crucial for comprehending the behavior of chemical compounds in various contexts.
In this article, we will delve into the concept of pKa, its significance, and methods for determining the pKa of compounds. We will also examine functional groups and structural factors that influence pKa values and explore practical applications of compounds with low pKa values.
pKa Values: A Measure of Acidity
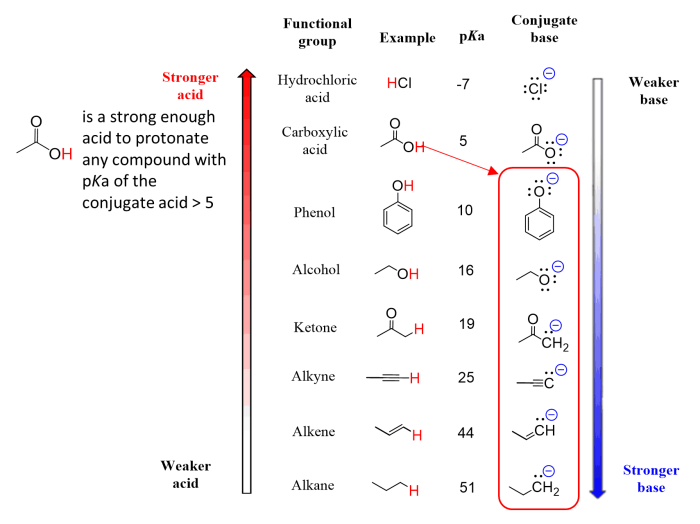
In chemistry, the pKa value is a measure of the acidity of a compound. It is defined as the negative logarithm of the acid dissociation constant (Ka), which is the equilibrium constant for the dissociation of an acid in water.
The lower the pKa value, the stronger the acid.
pKa values are important because they can be used to predict the behavior of compounds in chemical reactions. For example, compounds with low pKa values are more likely to donate protons, while compounds with high pKa values are more likely to accept protons.
Compounds with Low pKa Values
The functional groups that typically have low pKa values are those that can easily lose a proton. These include:
- Carboxylic acids
- Phenols
- Alcohols
- Amines
The electronic and structural factors that contribute to low pKa values include:
- Electronegativity of the atom bonded to the proton
- Resonance stabilization of the conjugate base
- Steric hindrance around the proton
Identifying Compounds with Lowest pKa
The pKa of a compound can be determined using a variety of methods, including:
- Potentiometric titration
- Spectrophotometry
- NMR spectroscopy
Once the pKa values of different compounds have been determined, they can be compared to identify the compound with the lowest pKa value.
For example, the pKa values of acetic acid, phenol, and ethanol are 4.76, 10.00, and 15.90, respectively. This indicates that acetic acid is the strongest acid of the three, followed by phenol and then ethanol.
Applications of Low pKa Compounds, Which of the following compounds has the lowest pka
Compounds with low pKa values have a wide range of applications, including:
- As acids in chemical reactions
- As buffers to control the pH of solutions
- As catalysts for chemical reactions
- As starting materials for the synthesis of other compounds
For example, acetic acid is used as a solvent, a food additive, and a starting material for the synthesis of other chemicals. Phenol is used as a disinfectant, an antiseptic, and a starting material for the synthesis of plastics. Ethanol is used as a solvent, a fuel, and a starting material for the synthesis of other chemicals.
Expert Answers: Which Of The Following Compounds Has The Lowest Pka
What is the significance of pKa in chemistry?
pKa is a crucial parameter that helps predict the reactivity and behavior of chemical compounds in various chemical and biological processes.
How can we determine the pKa of a compound?
Several methods can be employed to determine the pKa of a compound, including potentiometric titrations, spectrophotometry, and nuclear magnetic resonance (NMR) spectroscopy.