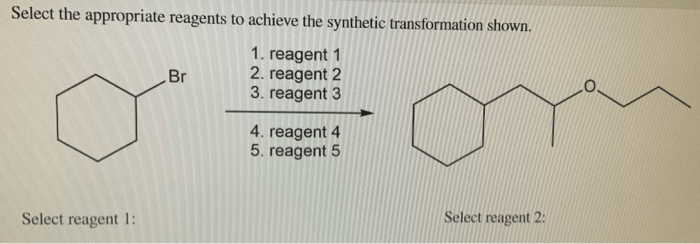
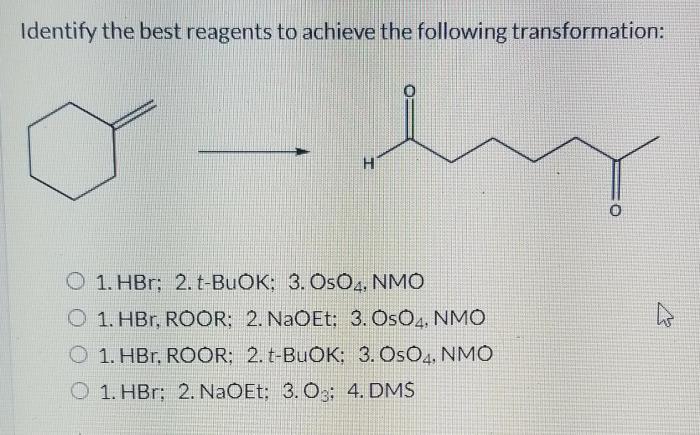
Select the appropriate reagents to achieve the synthetic transformation shown – Selecting the appropriate reagents is a crucial aspect of organic synthesis, directly influencing the efficiency, selectivity, and overall success of synthetic transformations. This article provides a comprehensive overview of reagent selection strategies, encompassing key considerations, reaction mechanisms, and practical applications.
By understanding the factors that govern reagent choice, chemists can optimize their synthetic routes, minimize waste, and achieve desired outcomes with greater precision.
Factors influencing reagent selection include functional group compatibility, reaction mechanism, and selectivity. Common reagents used in different types of synthetic transformations will be discussed, along with examples to illustrate their applications. Additionally, the role of retrosynthesis in planning synthetic strategies and identifying functional group interconversions will be explored.
1. Reagents Selection for Synthetic Transformations
Selecting appropriate reagents in organic synthesis is crucial for achieving desired outcomes efficiently and selectively. Key considerations include:
- Functional group compatibility: Reagents must be compatible with the functional groups present in the starting materials and products.
- Reaction mechanism: The choice of reagent depends on the reaction mechanism involved, such as nucleophilic substitution, electrophilic addition, or radical reactions.
- Selectivity: Reagents should exhibit high selectivity for the desired functional group transformation or reaction pathway.
Common reagents used in organic synthesis include:
- Nucleophiles: Grignard reagents, organolithium compounds, alkoxides
- Electrophiles: Alkyl halides, acyl chlorides, aldehydes, ketones
- Oxidizing agents: Potassium permanganate, sodium dichromate, hydrogen peroxide
- Reducing agents: Sodium borohydride, lithium aluminum hydride, hydrogen gas
2. Synthetic Strategies and Reagent Selection

Retrosynthesis is a powerful tool for planning synthetic transformations. It involves working backward from the target molecule to identify functional group interconversions and select reagents that facilitate these transformations.
A step-by-step approach to reagent selection based on synthetic goals:
- Identify the target molecule and its functional groups.
- Analyze the starting materials and identify the functional group transformations required.
- Research potential reagents that can perform the desired transformations.
- Consider the compatibility, selectivity, and efficiency of the reagents.
- Select the most appropriate reagents based on the above factors.
3. Reagent Reactivity and Selectivity
Reagent reactivity refers to its ability to undergo chemical reactions. Selectivity describes the reagent’s ability to react preferentially with a specific functional group or reaction pathway.
Types of selectivity in organic reactions:
- Chemoselectivity: Selectivity for one functional group over another.
- Regioselectivity: Selectivity for a specific position within a molecule.
- Stereoselectivity: Selectivity for a specific stereoisomer.
Examples of reagents that exhibit high selectivity:
- Osmium tetroxide for selective dihydroxylation of alkenes
- Potassium permanganate for selective oxidation of alkenes to diols
- Lithium diisopropylamide (LDA) for selective deprotonation of amides
4. Optimization and Troubleshooting
Optimizing reaction conditions is crucial to achieve desired outcomes. Troubleshooting strategies for reagent selection and synthetic transformations include:
- Adjusting reaction temperature, time, and solvent.
- Modifying reagent stoichiometry or concentration.
- Using additives or catalysts to enhance selectivity or efficiency.
Guidance on modifying reagents or reaction conditions to improve yield, selectivity, or efficiency:
- Increase reagent concentration or reaction time to improve yield.
- Use a more selective reagent to enhance selectivity.
- Add a catalyst to increase reaction rate and efficiency.
5. Applications in Chemical Synthesis
Appropriate reagent selection is essential for efficient and sustainable chemical synthesis. Examples of successful synthetic transformations that highlight the importance of reagent choice:
- Synthesis of pharmaceuticals: Taxol, a cancer drug, was synthesized using a complex series of reagents to achieve the desired stereochemistry and functionality.
- Synthesis of natural products: The total synthesis of morphine, a powerful painkiller, required careful selection of reagents to control regio- and stereoselectivity.
- Green chemistry: The development of environmentally friendly reagents and reaction conditions has led to more sustainable synthetic methods.
Common Queries: Select The Appropriate Reagents To Achieve The Synthetic Transformation Shown
What are the key considerations for selecting appropriate reagents in organic synthesis?
Key considerations include functional group compatibility, reaction mechanism, selectivity, cost, availability, and safety.
How does retrosynthesis help in selecting reagents?
Retrosynthesis involves working backward from the target molecule to identify the starting materials and reagents needed. This approach helps identify functional group interconversions and select reagents that facilitate these transformations.
What is the difference between chemoselectivity, regioselectivity, and stereoselectivity?
Chemoselectivity refers to the selective reaction of a reagent with one functional group over others. Regioselectivity involves the selective reaction at a specific site within a molecule. Stereoselectivity controls the formation of specific stereoisomers.